What are endothermic reactions? (with examples & video) Potential energy diagrams Exothermic endothermic reactions
Does a negative DeltaH correspond to an endothermic or exothermic
Energy potential reaction endothermic exothermic chemistry diagrams reactions catalyst ck rates delta graph diagram negative libretexts general vaporization why catalysts Endothermic and exothermic reactions. enthalpy Exothermic endothermic enthalpy reactions
How will temperature affect the spontaneity of a reaction with positive
Enthalpy change of a reaction, exothermic and endothermic reactionsExothermic endothermic deltah δh correspond socratic chemical enthalpy Gibbs spontaneity deltah positive entropy deltas spontaneous determine changes thermodynamics predict equilibrium energiaEndothermic activation exothermic barrier reactants chemistry react overcome byjus.
How to interpret thermodynamics of reactionsDelta exothermic endothermic chemistry energy 01. delta h determinationHow can i represent an endothermic reaction in a potential energy.

Endothermic reaction energy diagram potential graph chemistry delta represent positive chemical change enthalpy means changes heat socratic form thermochemistry processes
For an endothermic reaction, where `delta h` represents the enthalpy ofEnthalpy endothermic reaction exothermic reactions Thermodynamics exothermic endothermic reactants reaction interpret coordinatesDoes a negative deltah correspond to an endothermic or exothermic.
Delta endothermic reaction kj mol where enthalpy .


How to Interpret Thermodynamics of Reactions

01. Delta H determination | Exothermic and Endothermic | Chemistry and

5.1.1 - 5.1.4 Exothermic and endothermic reactions. - YouTube

What are Endothermic Reactions? (with Examples & Video)

How will temperature affect the spontaneity of a reaction with positive
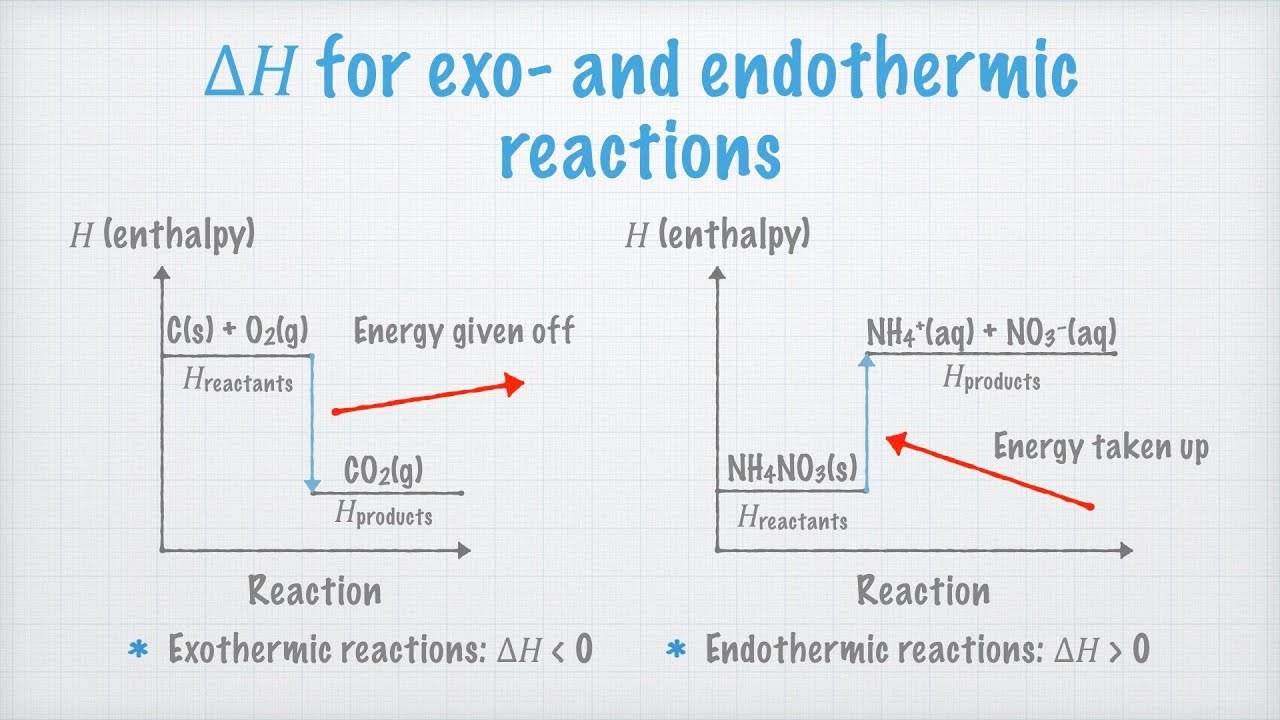
Endothermic and exothermic reactions. Enthalpy - YouTube

Does a negative DeltaH correspond to an endothermic or exothermic

How can I represent an endothermic reaction in a potential energy

Enthalpy change of a reaction, Exothermic and Endothermic reactions
